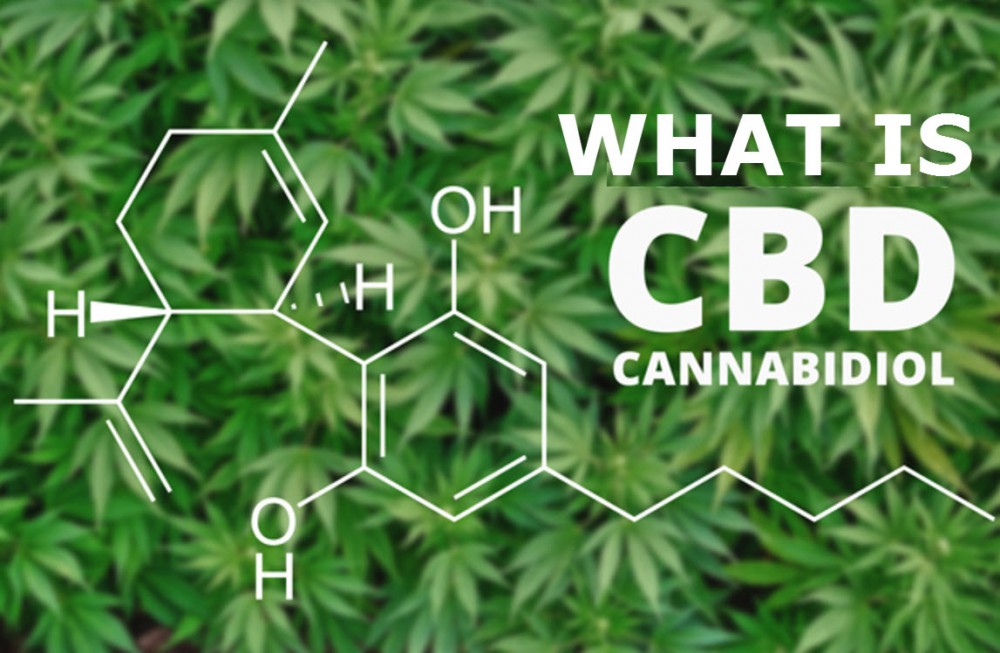
World Health Organization: CBD Has No Health Risks
World Health Organization Says CBD has No Health Risks from CannabisNet on Vimeo.
The United Nations’ health agency, the World Health Organization, has just given CBD the green light by officially saying that it shouldn’t be internationally controlled. The WHO’s findings were reviewed and discussed as part of their annual meetings held each November.
The WHO report clearly said that they find CBD has no potential health or abuse risks and instead backed up existing scientific evidence by saying that CBD has therapeutic applications. The report also acknowledges that CBD is a naturally occurring compound, that it is safe in both humans and animals, and has not been linked to any negative side effects. Additionally, the WHO writes that unlike THC, users can’t get high from CBD.
“In an animal drug discrimination model, CBD failed to substitute for THC. In humans, CBD exhibits no effects indicative of any abuse or dependence potential,” says the report. “To date, there is no evidence of recreational use of CBD or any public health related problems associated with the use of pure CBD.” The report echoes other scientific and medical reports proving the efficacy of CBD in easing the effects of THC such as its psychoactive properties or the anxiety it causes in some users.
The WHO Expert Committee on Drug Dependence (ECDD) stated in an announcement: “Recent evidence from animal and human studies shows that its use could have some therapeutic value for seizures due to epilepsy and related conditions. Current evidence also shows that cannabidiol is not likely to be abused or create dependence as for other cannabinoids (such as Tetra Hydro Cannabidiol [THC], for instance.”
The WHO report also acknowledges the therapeutic benefits of CBD for treating Crohn’s disease, Alzheimer’s disease, Parkinson’s disease, certain kinds of cancer, and other chronic and life-threatening conditions.
The report also discusses the development of science-backed evidence of CBD as medicine and its impact on international regulations, saying: “Several countries have modified their national controls to accommodate CBD as a medicinal product.” However, the United States isn’t one of them; hemp-derived CBD is a Schedule 1 substance but is processed and sold without any regulation. However, the study says that CBD from industrial hemp contains less than .3% THC basically doesn’t have any psychoactive effects.
Additionally, the WHO committee announces that they have plans to take an even deeper look into CBD by next year:
“There is increased interest from Member States in the use of cannabis for medical indications including for palliative care. Responding to that interest and increase in use, WHO has in recent years gathered more robust scientific evidence on therapeutic use and side effects of cannabis and cannabis components.
To that end, the ECDD did an initial review of a cannabis compound called cannabidiol (CBD). Recent evidence from animal and human studies shows that its use could have some therapeutic value for seizures due to epilepsy and related conditions. Current evidence also shows that cannabidiol is not likely to be abused or create dependence as for other cannabinoids (such as Tetra Hydro Cannabidiol (THC), for instance). The ECDD therefore concluded that current information does not justify scheduling of cannabidiol and postponed a fuller review of cannabidiol preparations to May 2018, when the committee will undertake a comprehensive review of cannabis and cannabis related substances.”
The 40th ECDD dialogue is set for May 2018.
Lack Of Regulatory Oversight
Even though hemp-derived CBD is accessible online and in several US states, a November 2017 report revealed that almost 70% of CBD extracts are mislabeled. The study, which looked at 31 companies that manufactured 84 CBD products, showed that only around 31% of the items contained accurate amounts of CBD – the same levels of CBD that were listed on its label.
Some of the CBD products were found to contain THC, which is not good news. The study’s authors recommend that CBD extract manufacturers require improved quality control and regulatory oversight so that consumers are getting accurate amounts of unadulterated CBD.
“People are using this as medicine for many conditions (anxiety, inflammation, pain, epilepsy),” says study author Marcel Bonn-Miller of the University of Pennsylvania Perelman School of Medicine. “The biggest implication of the study is that many of these patients may not be getting the proper dosage; they’re either not getting enough for it to be effective or they’re getting too much.”
Despite the fact that there are no negative side effects associated with consuming too much THC, we can all agree that it’s critical for companies to be transparent with their labeling otherwise these small issues will continue to attract reefer madness instead of the serious medical attention it should be receiving instead.
OTHER STORIES YOU MAY ENJOY...
WHY THE WORLD NEEDS MARIJUANA, CLICK HERE.