What You Need To Know About Dsuvia, Another Deadly But Recently Approved Opioid By The FDA

Hint: It’s 10 times stronger than fentanyl
The United States is drowning in an alarming opioid crisis which the government hasn’t seem to found a way out of. Legalizing cannabis has proven to help address the opioid crisis where it’s available, but when the government continues approving deadly opioids left and right, we’re just going to continue shooting ourselves in the foot now, aren’t we?
Early in November last year, the Food and Drug Administration (FDA) announced the green-light for Dsuvia, an opioid painkiller created to help patients manage acute pain in a medically supervised setting.
ACUTE PAIN??
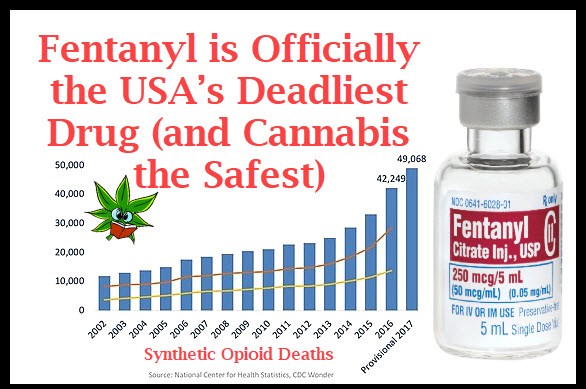
You’re better off with cannabis to treat any kind of pain. There is no need to resort to a deadly, addictive painkiller for that.
“The FDA approval of Dsuvia is the culmination of nearly 15 years of research to improve the standard of care for managing acute pain in medically supervised settings,” says Pamela Palmer MD, PhD, co-founder of Dsuvia manufacturer AcelRX and the company’s chief medical officer. “As an anesthesiologist, I’ve seen the challenges that IV opioids pose to patients and providers, such as logistical delays in initiating IV lines, difficulty in accessing veins and medication errors with injectable opioids,” reads the press release.
Dsuvia (sufentanil sublingual tablet), is a single-strength tablet packaged individually which they claimed would reduce the risk for inaccurate dosing and misuse.
You may not have heard of Dsuvia just yet, but you’ve heard about the notorious and deadly effects of fentanyl, an opioid that has been making the rounds throughout the country and taking lives in its wake. Fentanyl is 100 times stronger than morphine, and up to 80 times more powerful than heroin. In other words, anyone who takes fentanyl can easily overdose.
With the FDA’s approval of Dsuvia, this may lead to a slew of even more cases of addiction and overdose. The fact that it can only be given in a supervised setting doesn’t actually eliminate any of its risks involved. Just look at the stats: In 2017, prescription opioids were a main contributor to the opioid epidemic in the country, accounting for over 35% of opioid related deaths. Every single day, 46 people die from overdoses due to prescription opioids.
AcelRX claims in its press release, “Dsuvia will not be available in retail pharmacies or for outpatient use. Dsuvia will only be distributed to health care settings certified in the Dsuvia Risk Evaluation and Mitigation Strategy (REMS) program following attestation by an authorized representative that the healthcare setting will comply with appropriate dispensing and use restrictions of Dsuvia.”
There are many issues with AcelRX’s approach to “managing” how Dsuvia is consumed. First off, it’s assuming that everyone will comply with the guidelines without addressing the possibility that the drug can leak into the streets. Another issue is that it still doesn’t take into consideration the addictive potential of this drug EVEN within the hospital setting. Opioids are naturally addictive, and people can easily get addicted even if they take it following doctors’ prescriptions.
Straight From The Horse’s Mouth
In October last year, Dr. Raeford Brown of the FDA Anesthetic and Analgesic Products Advisory Committee expressed his doubts that the agency can efficiently “regulate Dsuvia so that it is used only in closely controlled settings” and “only as described in the label.” He explained that based on historical evidence, the FDA doesn’t have the ability to regulate these controls as observed with other opioids in the market, and “there is currently no educational nor regulatory scheme” that ensures the FDA approach to Dsuvia would actually work this time.
“It is my observation that once the FDA approves an opioid compound there are no safeguards as to the population that will be exposed, the post-marketing analysis of prescribing behavior, or the ongoing analysis of the risks of the drug to the general population,” he says in a letter co-signed by members of Public Citizen, a consumer advocacy group.
Meanwhile, the FDA is taking their sweet time figuring out how to regulate a federal CBD market. Or are they delaying it on purpose? They’re still spending so much time issuing letters to companies who are making CBD products while claiming that it can treat diseases, a statement that hasn’t been verified by federal agencies.
Focus on developing the regulatory framework, instead!
OTHER STORIES YOU MAY ENJOY...
FENTANYL IS DEADLY, AND CANANBIS IS SAFE, CLICK HERE.