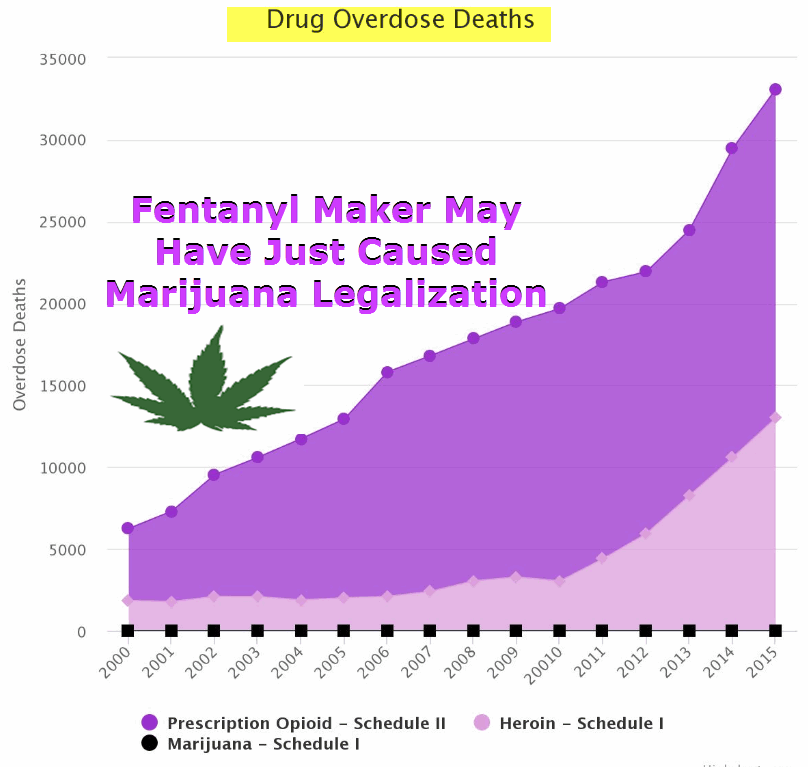
Fentanyl Maker Who Faked Caner Patients And Backed Arizona Prohibition In Trouble Again
Well, you know what they say: Karma’s a bitch.
Insys Therapeutics, the Arizona-based pharmaceutical company behind the deadly opioid Fentanyl is making headlines again, and it’s not a good thing for them.
Charges were filed last month by Arizona Attorney General Mark Brnovich, also the state’s top prosecutor, for Insys’ fraudulent marketing tactics just to earn profits from Subsys, a spray version of Fentanyl. The lawsuit states that Insys Therapeutics used a deceptive scheme to trick doctors, patients, and insurers about the safety of Subsys.
“Insys lied to insurers, concealed key facts from doctors and patients, and paid doctors sham ‘speaker fees’ in exchange for writing prescriptions, all in order to increase the sales of Subsys, without regard for the health and safety of patients,” Brnovich said. “Insys made hundreds of millions of dollars from its deceptive scheme, but also put countless patients in harm’s way, exposing them to unacceptable and unnecessary risks of addiction and death.”
Through Arizona’s Consumer Fraud Act, Brnovich is requesting a judge to prevent Insys from using misleading and unfair acts. Doing so enables Brnovich to demand Insys to pay restitution to patients who shouldn’t have been prescribed with Subsys , and demand the company to forfeit their profits from the illegal trade. Although Insys hasn’t commented, they definitely know that they’re under investigation.
If Brnovich’s claims are correct, this means that Insys paid off and lied to patients, insurance companies, and doctors while donating money to prevent people from obtaining the medicine that they need the most.
Faking Cancer Patients
US Senator Clair McCaskill released a new report discussing the elaborate scheme of Insys in faking disease diagnoses just to increase profits for cancer pain medication. According to the senator’s report, Insys manipulated insurer approval processes, bribed doctors, and faked patient files just to get more doctors to prescribe fentanyl to patients who didn’t even need it.
The Sept. 6 report entitled “Fueling an Epidemic: Insys Therapeutics and the Systemic Manipulation of Prior Authorization”, McCaskill investigated the pharmaceutical company as a means of better understanding the role of other pharmas in contributing to the current opioid epidemic. The report includes information taken from criminal and civil lawsuits as well as investigations of sales and marketing activities that Insys engaged in to sell Subsys, which was approved for cancer patients over the age of 18, by the US FDA. Subsys is a costly medicine that requires patients to get approval from insurance companies before doctors can prescribe it.
The report states, “According to public reporting, lawsuits from Subsys patients, and criminal indictments, Insys Therapeutics has repeatedly employed aggressive and likely illegal techniques to boost prescriptions for its fentanyl product Subsys.” Based on the findings, the illegal activities started in 2014.
McCaskill’s report even states that Insys established a special internal unit whose sole task was to contact insurance companies to get the prior authorization, a process wherein doctors have to obtain insurance approval for expensive medicine before they prescribe it. This system is meant to make sure that the patient has considered alternative treatments if any. Typically, doctors’ offices call insurance companies to get the approval. Insys worked with dishonest doctors and drug repos to convince insurers that the patients had cancer pain even if they did not have cancer. In doing so, they avoided using the word “cancer” but in the diagnosis, implied it. Insys even went great lengths to use a phone number designed to block caller ID’s so that insurers wouldn’t have a clue that the process has been controlled.
McCaskill’s office even furnished the report with a recording of a fraudulent call, where the Insys employee doesn’t disclose that she works for them but pretends to call the insurer from a doctor’s office. On the recording, the Insys employee continuously used the term “breakthrough pain” when she was asked about the diagnosis, but refrained from using the word “cancer”. The patient at the time, Sarah Fuller, was not diagnosed with cancer. She overdosed on Subsys and died in 2016.
Insys Backed AZ To Oppose Legalization
If Insys rings a bell, that’s because it’s the same company who donated $500,000 to prevent the passage of Prop 205 in Arizona last year, which would have made it legal to possess, grow, and sell cannabis in the state. Unfortunately, it was defeated 52-48. Insys has also received Schedule II approval for a synthetic THC product called Syndros, intended to treat cancer and AIDS patients for nausea, vomiting, and weight loss. On the other hand, cannabis remains on the Schedule I act, which is a much stricter category.
Insys has been active in fighting cannabis legalization for a long time now. Back in 2011, they even wrote to the DEA to oppose looser restrictions on natural THC, stating that “the abuse potential in terms of the need to grow and cultivate substantial crops of marijuana in the United States.” More recently they also petitioned the DEA to loosen restrictions on synthetic CBD, which of course would only benefit them since they are working on a CBD-based drug for pediatric epilepsy.
Insys Therapeutics is pure evil.
OTHER STORIES YOU MAY ENJOY...
CANNABIS FENTANYL AND WHY INSYS IS EVIL, READ THIS..